Start studying Protons Neutrons Electrons. Which of the following statements isare correct.

Atom Quiz Properties Of Protons Neutrons And Electrons Proprofs Quiz
The positive charge of the protons in the nucleus is balanced by the negative charge of the electrons.

. This is a tiny dense region at the center of the atom. A proton is one of three main particles that make up the atom. As a result a neutral atom must have an equal number of protons and electron.
P with a positive electric charge of 1e elementary charge and a mass slightly less than that of a neutron. A proton has a positive charge and is one of two types of particles that possess a greater amount of mass in an atom than electrons. A proton is a subatomic particle with a positive electric charge.
Electrons have a negative charge. Its got a charge of 1 and normally found inside the nucleus. Protons are charged particles.
Have a positive charge. Being found in the atom s centre or nucleus p rotons and neutrons are also known as nucleons. Learn vocabulary terms and more with flashcards games and other study tools.
Additionally what is the mass and charge of a proton. They are found in the nucleus along with neutrons. An atom is composed of protons electrons and neutrons.
Question 1 32 out of 32 points Protons have a _____ charge and are found in _____. If an ion has a 2 charge like Zn 2 this means there are two more protons than electrons. Protons are found in the nucleus of every atom.
Protons have a positive electrical charge of one 1 and a mass of 1 atomic mass unit amu which is about 1671027 kilograms. The charge on the proton and electron are exactly the same size but opposite. The charge is believed to be from the charge of the quarks that make up the nucleons protons and neutrons.
What made up the necuels. However protons have a charge of 1 and neutrons are uncharged. What type of electrical charge do protons have.
Scientists ideas about atoms have changed over time. The nucleus has a positive charge because neutrons have no electric charge. Summary Students will put a static charge on a strip of plastic by pulling it.
Electrons revolve around the nucleus in. Since opposite charges aract protons and electrons aract each other. Ii Its mass number is 23.
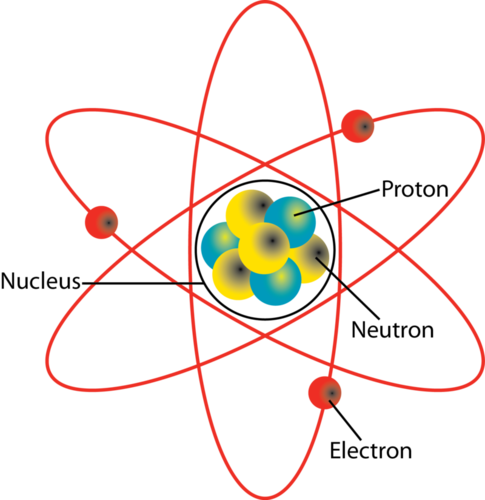
Protons and neutrons are located inside the nucleus of an atom. What do protons and neutrons have in common. Whereas electrons revolve around the nucleus.
What type of electrical charge do neutrons have. Neutrons do not have a net electric charge so the number of neutrons does not matter in the calculation. Protons have a positive charge.
For example a lithium atom Z3 A7 amu contains three protons found from Z three electrons as the number of protons is equal to the number of electrons in an atom and four. Protons and neutrons are found in the nucleus. The entire mass of an atom is concentrated in the nucleus which is at the centre.
Today they agree that atoms have a positively-charged nucleus made of protons and neutrons. And each electron has a negative charge. The nucleus is made out of protons positively charged particles and neutrons no charged.
The positive charge on a proton is equal in magnitude to the negative charge on an electron. Do protons have mass yes or no. The nucleus contains protons and neutrons which are collectively called nucleons.
Neutrons have no charge. Protons have a positive charge of 1. A modern perspective has a proton composed of the valence quarks up up down the gluons and transitory pairs of sea quarks.
The number of protons of an atom cannot change via any chemical reaction so you add or subtract electrons to get the correct charge. Protons have a positively charged and electrons are negatively charged. Are found in the nucleus.
Protons have a positive charge. In fact the number of protons in each atom is its atomic number. Small subatomic particles found outside the nucleus of atoms.
Protons are particles found in the area known as the atom s nucleus which concentrates almost all the atom s mass. The two up quarks and one down quark of a proton are held together by the strong force mediated by gluons. Protons are spin-12 fermions and are composed of three valence quarks making them baryons a sub-type of hadrons.
Neutrons are neutral particles. Protons are a type of subatomic particle with a positive charge. I This atom has an atomic number of 12.
Electrons have a mass of approximately 0 amu orbit the nucleus and have a charge of -1. An atom contains 11 protons and 12 neutrons in its nucleus. Electrons have a negative charge.
Protons are bound together in an atoms nucleus as a result of the strong nuclear force. What is a proton and its charge. Each proton carries a positive charge and each neutron has no charge.
Protons have a positive charge distribution which decays approximately exponentially with a mean square radiusof abou. E charge on the proton and electron are exactly the same size but opposite. A proton is a subatomic particle symbol.
A proton has a charge of 1 or 1e which is equal to 1602 x 10-19. Until recently the proton was considered a fundamental particle. Protons are the positively charged particles which are present in the nucleus of a hydrogen atom.
Positive the nucleus of an atom. Protons are found in the nucleus of the atom. Therefore we can conclude that Protons.
Protons are found in the nucleus of every atom but can also be found away from the nucleus most commonly as ionized hydrogen. Protons and neutrons have almost the same mass and are both located in the nucleus of the atom. They group together in the center of the atom.
A proton is made of two Up quarks with 23 positive charge each and one Down Quark with a negative 13 charge 23 23 -13 1. Also to know what is charge of electron and proton.

Protons Neutrons And Electrons Chapter 4 The Periodic Table Bonding Middle School Chemistry

Electricity And Magnetism Protons Have A Charge Electrons Have A Charge Most Atoms Are N Kwl Chart What Is Electricity Nonrenewable Resources

Atomic Structure Boundless Microbiology
What Two Particles Are Found In The Nucleus Of An Atom Socratic
0 Comments